Featured Companies
China Pharma, CPHI, Profile, Summary

China Pharma| CPHI| Profile| Summary
China Pharma, CPHI, is a specialty pharmaceutical company that develops, manufactures and markets generic and brand pharmaceutical products for both western and traditional Chinese medicines in China. Currently, the company’s main focus for its therapeutics includes CNS, cardiovascular, cerebro-vascular, wound recovery, digestive disease, and infectious diseases. Its products are sold in 30 provinces, municipalities, and autonomous regions. The Company has 16 sales offices and approximately 680 proxy agents in the whole country.
China Pharma is located in the tax-free industrial zone of Haikou, Hainan Province, with manufacturing facility of 8,000 square meters. The Company is equipped with eight GMP(Good Manufacturing Practice) certified production lines and has the capacity to produce drugs in a variety of delivery mechanisms including freeze dried(lyophilized injectables), capsules, tablets, granules, injectables, and oral fluids(suspension). The company is positioning itself to benefit from China’s rapidly growing pharmaceutical market as it expands its product portfolio.
Research & Development
China Pharma develops generic products based on market demand by combining internal product development, strategic alliances with partners and collaborations of its products and businesses. The Company targets off-patent drugs with cumulative global sales over $1billion. Under Chinese law, China Pharma is able to obtain exclusivity protection for three or more years for new generics it brings to market. The disease areas China Pharma is currently focusing on are: neurology, hyperlipidemia and cardiovascular disease, hyperglycemia and diabetes , hypertension, and infectious diseases. China Pharma has built strong research and development partnerships with the following elite institutions in China and retains the ownership rights to all resulting intellectual property of products.
Modern Manufacturing Facilities
China Pharma has scalable GMP-certified manufacturing facilities with eight different modern production lines. These facilities passed the GMP-certification when it was first introduced in 2003 and passed the latest round in 2008. The GMP-certification is repeated every five years.
In 2008 the Company invested $3.0 million to increase capacity of the dry powder line. China Pharma’s cutting edge production lines can manufacture products in the following forms of delivery: tablets; capsules; injectables; granules; oral fluids (suspension); and freeze dried (lyophilized injectables). Its production capacity fully satisfies the needs of the current portfolio.
→ -The company received GMP certification in 2003
→ - Recognized as “The Best Enterprise for Storing SARS Medicine” by Hainan Food and Drug Administration.
→ -Received national key new products certificate for Buflomedil Hydrochloride by the State Science and Technology Department, State Taxation Bureau, Ministry of Commerce, State Bureau of Quality Supervision, Inspection and Quarantine, and State Environmental Protection Bureau.
→ -Designated as key technology project in Hainan for Buflomedil Hydrochloride by
Haikou Municipality.
Source: China Pharma, OxBridge Research, Daily Stock Deals, OTC King, PSM
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks. OTCKing.com is an OxBridge affiliate/partner website.
Disclosure/Disclaimer:- OxBridge Research publishes sponsored research reports, advertorials and corporate profiles on its portal and several other websites/blogs, including this website/blog, owned and operated by OxBridge and/or its affiliates. OxBridge Research is not a Broker Dealer or a Registered Financial Adviser in any jurisdiction, whatsoever. All the information published on its website(s) and/or distributed to its members via various electronic means is for general awareness and entertainment purpose only. OxBridge urges investors to do their own due diligence and consult with their financial adviser prior to making any investment decision. We are expecting a payment from the company/a third party/shareholder. We receive compensation from companies for providing various IR services, including publication, advertisement,and social media awareness, therefore our views/opinion are inherently biased. Please read the full disclosure/disclaimer, if you need assistance contact This e-mail address is being protected from spambots. You need JavaScript enabled to view it
OxbridgeResearch.com, All Rights Reserved. Trademarks/logos are of their respective owners.
It's YOUR money - Invest WISELY TM
AXN,Aoxing Pharmaceuticals,Profile,Summary

AXN| Aoxing Pharmaceuticals | Profile | Summary
Aoxing Pharmaceuticals (AXN) is a specialty pharmaceutical company engaged in research, development, manufacturing and distribution of a variety of narcotics and pain-management products.
Aoxing was Founded in 1655, more than 300 years of heritage and deep roots in traditional and contemporary Chinese medicine.
The company has China's largest research & development facility (over 300 acre campus) and most advanced manufacturing facility for highly regulated narcotic medicines. Over 500 employees are dedicated in research and development of drugs for pain management, neurological disorders and narcotics.
Its facility is one of the few GMP facilities licensed for the manufacture of highly regulated narcotic medicines by the China State Food and Drug Administration (SFDA). It has strategic alliance partnerships with global pharmaceutical companies like the UK's Johnson Matthey Plc (LSE: JMAT), QRx Pharma (ASX: QRX) of Australia and others. Aoxing is based in China and its US office is located in Jersey City, NJ.
Source: Aoxing Pharmaceuticals, OxBridge Research, Daily Stock Deals, OTC King. PSM
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks. OTCKing.com is an OxBridge affiliate/partner website.
Disclosure/Disclaimer:- OxBridge Research publishes sponsored research reports, advertorials and corporate profiles on its portal and several other websites/blogs, including this website/blog, owned and operated by OxBridge and/or its affiliates. OxBridge Research is not a Broker Dealer or a Registered Financial Adviser in any jurisdiction, whatsoever. All the information published on its website(s) and/or distributed to its members via various electronic means is for general awareness and entertainment purpose only. OxBridge urges investors to do their own due diligence and consult with their financial adviser prior to making any investment decision. We are expecting a payment from the company/a third party/shareholder. We receive compensation from companies for providing various IR services, including publication, advertisement,and social media awareness, therefore our views/opinion are inherently biased. Please read the full disclosure/disclaimer, if you need assistance contact This e-mail address is being protected from spambots. You need JavaScript enabled to view it
OxbridgeResearch.com, All Rights Reserved. Trademarks/logos are of their respective owners.
It's YOUR money - Invest WISELY TM
GenVec, GNVC, Profile, Summary
GenVec | GNVC | Profile | Summary
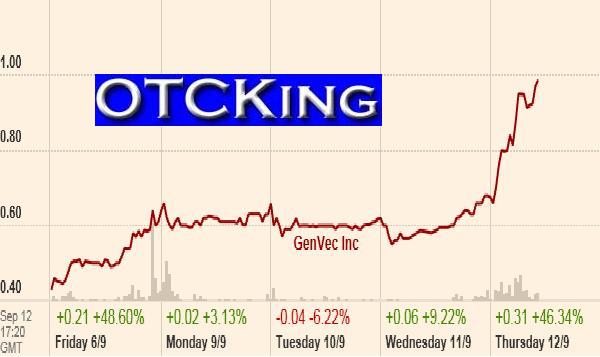
GenVec, (GNVC) is a biopharmaceutical company using differentiated, proprietary technologies to create superior therapeutics and vaccines. A key component of the company's strategy is to develop and commercialize products through collaborations. GenVec is working with leading companies and organizations such as Novartis, Merial, and the U.S. Government to support a portfolio of product programs that address the prevention and treatment of a number of significant human and animal health concerns.
GenVec’s development programs address therapeutic areas such as hearing loss, balance disorders, and cancer; as well as vaccines against infectious diseases including respiratory syncytial virus (RSV), herpes simplex virus type 2 (HSV-2), dengue fever, influenza, malaria, and human immunodeficiency virus (HIV).
The GenVec's Advantage
The company's core technology has the important advantage of localizing protein delivery in the body. This is accomplished by using adenovector platform to locally deliver genes to cells, which then direct production of the desired protein. This approach reduces side effects typically associated with systemic delivery of proteins. For vaccines, the goal is to induce an immune response against a target protein or antigen. This is accomplished by using an adenovector to deliver a gene that causes production of an antigen, which then stimulates the desired immune reaction by the body.
GenVec’s novel proprietary adenovectors
No pre existing immune recognition
Built on rare human and nonhuman adenovirus serotypes
New GC technology shows superior performance for genetic vaccines
Multiple capsid modified vectors -AdH technology
Multiple genes/multiple antigens
Targeted and specific delivery Proprietary adenovectors and cell lines
Novel proprietary non replicating adenovirus vectors
Very low sero -prevalence in humans
Outstanding gene/antigen delivery properties
Cell line master banks and master file with FDA
Hearing Restoration Molecular Therapeutic
Dengue Virus Genetic Vaccine
Foot and Mouth Virus Genetic Vaccine
Malaria Genetic Vaccine
RSV Genetic Vaccine
HSV Molecular Therapeutic and Genetic Vaccine
A platform of adenovirus vectors built to meet
product needs for molecular therapeutics and
genetic vaccines
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks. OTCKing.com is an OxBridge affiliate/partner website.
Disclosure/Disclaimer:- OxBridge Research publishes sponsored research reports, advertorials and corporate profiles on its portal and several other websites/blogs, including this website/blog, owned and operated by OxBridge and/or its affiliates. OxBridge Research is not a Broker Dealer or a Registered Financial Adviser in any jurisdiction, whatsoever. All the information published on its website(s) and/or distributed to its members via various electronic means is for general awareness and entertainment purpose only. OxBridge urges investors to do their own due diligence and consult with their financial adviser prior to making any investment decision. We are expecting a payment from the company/a third party/shareholder. We receive compensation from companies for providing various IR services, including publication, advertisement,and social media awareness, therefore our views/opinion are inherently biased. Please read the full disclosure/disclaimer, if you need assistance contact This e-mail address is being protected from spambots. You need JavaScript enabled to view it
OxbridgeResearch.com, All Rights Reserved. Trademarks/logos are of their respective owners.
It's YOUR money - Invest WISELY TM
Teryl Resources, TRC.V/TRYLF, Profile
Teryl Resources | TRC.V/TRYLF | Profile
Teryl Resources Corp., - TRC.V / TRYLF – has several gold prospects near the Kinross Gold Corp's Fort Knox Mine in Alaska, a silver/lead/zinc prospect adjacent to Silvercorp's (SVM) mine in British Columbia, and Oil & Gas wells in Texas.
Teryl has a 10% net profit interest in the Stepovich claims. A 100% interest in the Westridge property and a 50% interest in the Fish Creek property, adjacent to the Gil property. Teryl sold its 20% interest in the Gil property in Fairbanks, Alaska, to Fairbanks Gold Mining Corp., a wholly owned subsidiary of Kinross Gold Corp. (KCG), for $15 million dollars plus a ½ of 1% royalty for the life of the mine.
Teryl owns a 30% working interest and a 10% NPI interest in the Silverknife property, a silver/lead/zinc prospect located in Northern B.C. adjacent to Silvercorp's silver/lead/zinc discovery.
Teryl also has three producing oil and gas wells in Texas, these wells are operated by Anadarko Petroleum.
Fish Creek property
Teryl Resources has commenced a placer and hard rock diamond drilling program on the Fish Creek property in the Fairbanks, Alaska mining district.
Teryl's consulting geologist, Paul D. Gray, P.Geo. will oversee the drilling program, core logging , and assay protocols on behalf of the Company. The operator of the drill will be Kenn Roberts of Texas, and Pete Rutledge, project manager.
Kenn Roberts, a First Nations from Yukon has multiple years of drilling experience in remote locations and has served as mineral development advisor to First Nations.
Teryl owns a 50% interest in the Fish Creek property, Keltic Bryce owns a 50% working interest and Linux Gold Corp. (LNXGF) retains a 5% royalty interest in Teryl's interest.
The Fish Creek property consists of 30 claims adjacent to the Gil property, currently owned by Kinross Gold Corporation. The Gil 20% interest, previously owned by Teryl Resources Corp., was acquired by Kinross for $15 million dollars.
Westridge Property
A geological report on the Westridge gold property has been prepared by David Adams, B.S., M.S., CPG. Teryl has appointed Pete Rutledge, Geologist, as an independent contractor to evaluate the Westridge property.
The Westridge property is located approximately 16 km north of Fairbanks, Alaska. The property consists of 48 State of Alaska mining claims controlled by Teryl Resources Corp.
The claims cover approximately 1,749 acres on the north flank of the main ridge between Pedro Creek and upper Dome Creek; both of these drainages were significant historic placer gold producers. Access to the property is excellent: the Elliott Highway transects the westernmost edge of the property, and the Silver Fox Mine road transects the southwest portion of the property.
All claim holdings comprising the property are in good standing, and no encumbrances to future mining activities are known or anticipated.
SILVERKNIFE PROPERTY OWNERSHIP:
» Teryl Resources Corp. owns 30% working
interest and has a 10% Net Profit Interest
(“NPI”)
» Minewest Silver & Gold owns 70% subject to
the 10% NPI held by Teryl
The Silverknife Property
(Mineral Claims consisting of 1,594 Acres)
A proposed Phase I exploration program consisting of a desk study followed by a series of on-the-ground Property boundary and drill collar location surveys, followed by geophysics and diamond drilling with a
recommended budget of $358,700 is recommended for the Silverknife Property.
The exploration programs (and budgets), presented herein, are designed to identify the accurate location of the mineral titles
boundary with respect to historic drill collars and test the Silverknife Property’s precious and base metal mineral potential and will yield enough information to guide Minewest and Teryl subsequent mineral
exploration programs on the Property. Samples have been collected to confirm the assay results, however, due to the inclement weather conditions, the Phase I exploration program has been delayed.
Sources: Teryl Resources Corp., Kinross Gold Corp., Linux Gold Corp., SilverCorp.
Forward-looking statements:- The Company's actual results, performance or achievements could differ materially from those expressed in, or implied by, these forward-looking statements, including those described in the Company's Financial Statements, Management Discussion and Analysis and Material Change Reports filed with the Canadian Securities Administrators and available at www.sedar.com, and the Company's 20-F annual report filed with the United States Securities and Exchange Commission at www.sec.gov.
This profile/research report/email letter/blog/posting in forums/social-media/t/f does not constitute an offer to sell or a solicitation of an offer to buy any of the securities in the United States. The securities of the Company have not been registered under the United States Securities Act of 1933, as amended (the "U.S. Securities Act") or any state securities laws and may not be offered or sold within the United States or to U.S. Persons unless registered under the U.S. Securities Act and applicable state securities laws or an exemption from such registration is available. Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
Disclosure/Disclaimer:- OxBridge Research publishes sponsored research reports, advertorials and corporate profiles on its portal and several other websites/blogs, including this website/blog, owned and operated by OxBridge and/or its affiliates. OxBridge Research is not a Broker Dealer or a Registered Financial Adviser in any jurisdiction, whatsoever. All the information published on its website(s) and/or distributed to its members via various electronic means is for general awareness and entertainment purpose only. OxBridge urges investors to do their own due diligence and consult with their financial adviser prior to making any investment decision. We are expecting a payment of up to five thousand dollars in compensation from the company/a third party/shareholder. We receive compensation from companies for providing various IR services, including publication, advertisement,and social media awareness, therefore our views/opinion are inherently biased. Please read the full disclosure/disclaimer, if you need assistance contact This e-mail address is being protected from spambots. You need JavaScript enabled to view it
OxbridgeResearch.com, All Rights Reserved. Trademarks/logos are of their respective owners.
It's YOUR money - Invest WISELY TM





 OTCKing
OTCKing Feed Entries
Feed Entries
