Featured Companies
Aethlon Medical, AEMD, Profile, Summary
Aethlon Medical, AEMD, Profile, Summary

Aethlon Medical (AEMD) is developing innovative medical devices to address unmet medical needs in cancer, infectious disease, and other life-threatening conditions. Aethlon’s ADAPT™ platform provides the technology foundation for a new class of therapeutics that target the selective removal of disease enabling particles from the entire circulatory system. The Aethlon ADAPT™ product pipeline includes the Hemopurifier®, a first-in-class medical device with broad-spectrum capabilities against exosomes that contribute to the progression of cancer and infectious viral pathogens such as HIV and Hepatitis C.
HER2 Protein and Breast Cancer
Breast cancer is the most common form of cancer in women, accounting for 20% of cancer deaths with approximately 180,000 women diagnosed annually in the United States (Merrill, R. M., and A. Sloan. 2011. Risk-adjusted female breast cancer incidence rates in the United States. Cancer Epidemiol.). Over-expression of the human epidermal growth factor receptor 2 (HER2; ErbB2) gene occurs in approximately 25% of breast cancers and has been linked to poor prognosis (Slamon, D. J., G. M. Clark, S. G. Wong, W. J. Levin, A. Ullrich, and W. L. McGuire. 1987. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177-182). HER2 is a receptor tyrosine kinase, member of the EGF receptor family, which possesses proliferative and anti-apoptotic activities.
The standard of treatment for women diagnosed with HER2+ breast cancer includes the humanized monoclonal antibody trastuzumab (marketed as Herceptin®) directed against the extracellular domain of HER2. Trastuzumab binding to HER2 mediates direct growthinhibition of tumor cells and induces antibody-dependent cell cytotoxicity (ADCC), a major anti-cancer mechanism by which natural killer (NK) cells lyse antibody-coated tumor cells (Nahta, R., D. Yu, M. C. Hung, G. N. Hortobagyi, and F. J. Esteva. 2006. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol 3:269-280).
In patients with metastatic breast cancer undergoing chemotherapy, treatment with trastuzumab augments overall response rates and increases median survival time by 25% (Baselga, J. 2001. Clinical trials of Herceptin® (trastuzumab). Eur J Cancer 37 Suppl 1:18-24). However, response rates to trastuzumab range from 12% to 34% with median duration of 9 months due to development of resistance. Despite co-treatment with other HER2 targeting therapies (e.g. the tyrosine kinase inhibitor lapatinib) and chemotherapy, patients with metastatic breast cancer experience a limited duration of benefit; therefore, novel treatment strategies that act synergistically or overcome drug resistance are urgently needed (Sachdev, J. C., and M. Jahanzeb. 2011. Blockade of the HER Family of Receptors in the Treatment of HER2-Positive Metastatic Breast Cancer.
The Evolution of Therapeutic Filtration to Improve Hepatitis-C Virus (HCV) Treatment Outcomes
Therapeutic filtration of HCV from the entire circulatory system with a medical device has been clinically demonstrated to improve treatment outcomes when administered at the outset of standard of care (SOC) drug therapy. This summary overview, which includes an introduction to the Aethlon Hemopurifier®, is for informational purposes only.
About HCV Infection
The U.S. National Institutes of Health (NIH) reports an estimated 180 million people worldwide are afflicted with chronic HCV infection. Of people infected, 55 to 85% of will develop chronic infection, and 75% of those will develop chronic liver disease. Standard of care (SOC) drug therapy is represented by a two drug combination of interferon and ribavirin. The primary goal of SOC drug therapy is the achievement of a sustained virologic response (SVR), defined as undetectable viral load six months after completion of SOC drug therapy. According to the NIH, 15-25% of HCV infected patients will recover
completely.
SOC Drug Therapy Outcomes
•The largest study of HCV infected individuals pursuing 48-week SOC drug
therapy was a study of 4,469 genotype 1 patients published by McHutchison in The New England Journal of Medicine in August of 2009.
•31% (1,399 of 4,469) of patients screened for participation were excluded from the study.
•54% (1,654 of 3,070) of treated patients completed the SOC treatment regimen.
•39.6% (1,215 of 3,070) of treated patients who completed SOC therapy
achieved a SVR.
•27% (1,215 of 4,469) of the total patients screened for study participation
achieved a SVR.
•No data recorded for patients who may have relapsed after achieving a SVR.
The Hemopurifier®
The first medical device to selectively remove HCV and related immunosuppressive proteins from circulation. A Study of the Hemopurifier® + SOC Drug Therapy is Now Underway
•Patient enrollment has been initiated at the Medanta Medicity Institute in India - www.medanta.org
•Additional details can be accessed from the Clinical Trials Registry - India (CTRI) link: http://ctri.nic.in/clinicaltrials/index.jsp
•Up to 30 patients / up to 6 treatments in first 3 days of SOC
•Early clinical endpoints include:
•Immediate Virologic Response (IVR)
•Rapid Virologic Response (RVR)
•Early Virologic Response (EVR)
The Hemopurifier® Other Selected Quick Facts
•70 human treatment experiences administered to date
•An Investigation Device Exemption (IDE) to initiate U.S. studies has been filed with FDA
•Substantial viral load reductions have also been observed in a human HIV study performed in the absence of antiviral drugs
•GMP manufacturing has been established in an FDA approved facility
•The Hemopurifier® has proven broad-spectrum capabilities against viral bioterror and pandemic threats
•The device has been discovered to capture tumor-secreted exosomes known to suppress the immune system of cancer patients
Sources: The Company, OxBridge Research, OTCKING, DailyStockDeals, OTCstockIQ
Don't miss the NEXT premium Alert! Sign-up, Get Alerts,MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you would like your company featured or want to learn more, please don't hesitate to contact the Editor. editor [@] OxBridgeResearch.com
ALS, Hepatitis C, Traumatic Brain Injury, Blood Sepsis,breast cancer,HIV,HPV,HCV,SOC, dialysis,DOD,UNT,USPTO, NFL,SOCCOR,MILITARY,COMBAT MISSIONS,PTSD, Ebola
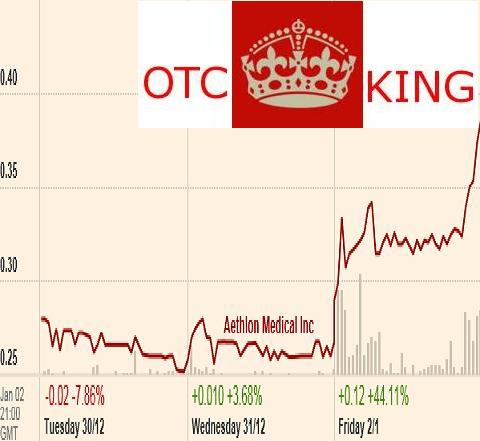




 OTCKing
OTCKing
