Featured Companies
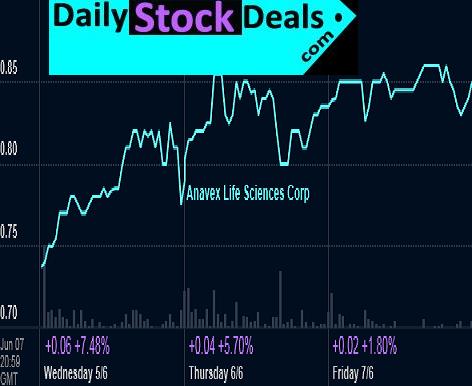
Anavex Life Sciences, AVXL, Profile, Summary
Anavex Life Sciences |AVXL | Profile
Anavex Life Sciences Corp. is a biopharmaceutical company engaged in the discovery and development of new drugs for the treatment of neurological diseases and cancer, utilizing its proprietary drug discovery SIGMACEPTOR™ platform. The Anavex portfolio comprises novel, wholly owned sigma receptor agonists and antagonists. The company’s lead drug candidate for Alzheimer’s Disease (AD), ANAVEX 2-73, has successfully completed a Phase 1 single ascending dose (SAD) clinical trial. The company has also started scale-up manufacturing for ANAVEX 1-41, its second lead compound, targeting depression and AD. With sufficient quantities of ANAVEX 1-41 in hand, the company will be in a position to advance the program and begin preclinical studies on large animals in the near term.
Neurological diseases (CNS): Anavex SIGMACEPTOR™-N program involves the discovery and development of original drug candidates, targeting neurological diseases such as Alzheimer’s disease, epilepsy, and depression. The company’s lead drug candidates exhibit high affinity and selectivity to sigma receptors and also synergistic action with other receptors, such as Muscarinic and NMDA, with strong evidence of anti-amnesic, neuroprotective, and anxiolytic properties.
Oncology: Anavex SIGMACEPTOR™-C program involves the discovery and development of original drug candidates targeting cancer. The company’s lead drug candidates exhibit high affinity to sigma receptors with strong evidence for selective apoptosis of cancerous cells, without affecting healthy cells, anti-metastatic and low toxicity properties in various types of cancer such as melanoma, prostate and pancreas, and neuropathic pain.
In addition, several series of sigma ligands currently at different stages of development, target stroke, diabetes, multiple sclerosis and Parkinson’s disease.
The Pipe Line
Anavex’s lead drug candidate ANAVEX 2-73 -targeting Alzheimer’s Disease- is in Phase 1 clinical trials. The company has also initiated scale-up manufacturing of ANAVEX 1-41 targeting a range of important CNS disorders, including depression and Alzheimer’s Disease. Three more of its compounds (for malignant melanoma, prostatic cancer and epilepsy) are expected to reach final preclinical stages in the near term.
Other early-stage preclinical research (see diagram) involves drug candidates that target diseases like depression, neuropathic pain and various types of cancer.
Source: Anavex, OxBridge Research, Daily Stock Deals
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
FFFC, Fast Funds Financial, Profile, Life Collateralized Mortgage Obligagtion
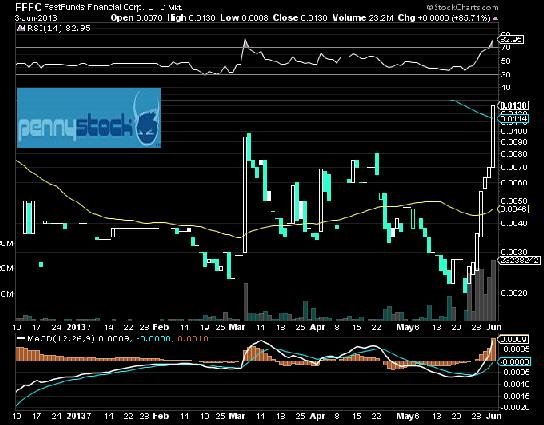
FastFunds Financial | FFFC | Profile
FastFunds Financial Corporation is a holding company currently operates in the financial services industry segment. The company's wholly-owned subsidiary, NET LIFE Financial Processing, Inc., has acquired the exclusive mortgage servicing rights for NET LIFE Financial Holdings ("NET LIFE") from the holder, a Florida Trust.
NET LIFE is a development stage enterprise that has developed and is offering an innovative new mortgage product that is not based on credit history (no doc) or personal guarantees. It is only secured by the underlying collateral and a life insurance policy on the borrower. Therefore, all that is required to qualify for a mortgage loan is qualifying for a life insurance policy, a down payment that usually amounts to 10% of the purchase price and verification that the borrower has the financial ability to pay the monthly payments. NET LIFE believes this mortgage product will be attractive to a wide spectrum of potential borrowers including:
first time home buyers
-
borrowers who have experienced prior financial difficulties such as foreclosures, bankruptcies, late payments or credit problems; presently employed and whose current income would qualify for a mortgage loan; but who couldn't otherwise qualify
-
borrowers who may wish to bypass the traditional paperwork involved in the typical underwriting process but who would otherwise qualify.
Innovative Mortgage Products
FastFunds Financial combined with its partner, NET LIFE, have developed an innovative new mortgage product that is not based on credit history (no doc) or personal guarantees. It is only secured by the underlying collateral and a life insurance policy on the borrower. Therefore, all that is required to qualify for a mortgage loan is qualifying for a life insurance policy, a down payment that usually amounts to 10% of the purchase price and verification that the borrower has the financial ability to pay the monthly payments. NET LIFE believes this mortgage product will be beneficial to a wide spectrum of potential borrowers including individuals and businesses.
NET LIFE LCMO
The Company believes a NET LIFE LCMO (Life Collateralized Mortgage Obligation) could substantially improve America's banking and mortgage crisis. A NET LIFE LCMO is a residential, business or corporate real estate mortgage which is not based on credit history; only on the collateral.
All that is required for which to qualify is:
(a.) that you are healthy
(b.) that the property maintains and sustains its stated value
(c.) that you have the income to pay your mortgage.
All NET LIFE LCMO mortgages are processed, managed and serviced exactly like a conventional mortgage without all of the red-tape qualification. NET LIFE has only reinvented the "vehicle"; not the "wheel" it rides upon.
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
Titan Pharmaceuticals, TTNP, Profile
Titan Pharmaceuticals,TTNP, Profile

Titan Pharmaceuticals, TTNP, is a biopharmaceutical Company developing proprietary therapeutics primarily for the treatment of serious medical disorders.
Titan’s principal asset is Probuphine®, the first slow-release implant formulation of buprenorphine hydrochloride (“buprenorphine”), designed to maintain a stable, round-the-clock blood level of the medicine in patients for up to six months following a single treatment. The outpatient treatment of opioid dependence with daily dosed sublingual buprenorphine formulations represents a $1.3 billion market in the U.S., and a seven day transdermal patch formulation of buprenorphine for the treatment of chronic pain was launched in the U.S. in 2011. This novel implant formulation is inserted subdermally in a patient’s upper arm providing continuous medication, and has the potential to enhance patient compliance to treatment, and limit diversion for illicit use and accidental exposure to the sublingual formulations.
The New Drug Application (NDA) was submitted to the FDA in October, 2012 seeking approval for treatment of opioid dependence. In December 2012, Titan entered into a license agreement with Braeburn Pharmaceuticals Sprl (“Braeburn”) that grants Braeburn exclusive commercialization rights to Probuphine® in the United States and Canada. Titan received a non-refundable up-front license fee of $15.75 million and will receive a $50 million milestone payment upon the approval of the NDA by the FDA. Additionally, Titan will be eligible to receive up to $130 million upon achievement of specified sales milestones and up to $35 million in regulatory milestones in the event of future NDA submissions and approvals for additional indications, including chronic pain. Titan will receive tiered royalties on net sales of Probuphine ranging from the mid-teens to the low twenties.
Probuphine is the first product to utilize ProNeura™, a novel, proprietary, long-term drug delivery technology. The ProNeura technology has the potential to be used in developing products for the treatment of other chronic conditions, such as Parkinson’s disease, where maintaining stable, round-the-clock blood levels of a drug can benefit the patient and improve medical outcomes.
Finally, Titan is also entitled to royalty revenue of 8-10% of net sales of Fanapt® (iloperidone), an atypical antipsychotic compound being marketed in the U.S. for the treatment of schizophrenia by Novartis Pharma AG (“Novartis”) under a sub-license agreement based on a licensed U.S. patent that expires in October 2016 (does not include a possible six month pediatric extension). Substantially all of this future royalty revenue has been sold to Deerfield Management (“Deerfield”), a healthcare investment fund, in exchange for cash and debt considerations which have been used to advance the development of Probuphine and for general corporate purposes.
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
Sources: company, OxBridge Research, Daily Stock Deals
GNIN, Green Innovations, Summary, Profile
GNIN, Green Innovations, Summary, Profile

Green Innovations Ltd is focused on filling a void in the eco-friendly, biodegradable product array. As the world continues to trend away from tree-based products in favor of products with smaller carbon footprints, consumers are becoming more educated about the “green” products that are available to them in the marketplace and are consequently demanding higher standards for these products. In order to help satisfy this demand, Green Innovations Ltd, through its wholly-owned subsidiary Green Hygienics Inc., is the exclusive and licensed North American distributor of American Hygienics Corporation 100% tree-free, bamboo-based product line, including personal care and paper-based goods.
Our products provide their end-users the opportunity to enjoy the quality and performance delivered by a company that has been producing for over a decade, the cost-benefit of a company that manufactures local raw material, and the satisfaction of knowing that by using these products they are doing their part to reduce their carbon footprint and to continue the movement towards a more healthy and sustainable planet.
Eco-Friendly Products
Bamboo products are far more earth-friendly than their conventional, paper-based alternatives. Bamboo can be grown and harvested in less than 5 years, during which time the bamboo will absorb more carbon dioxide and release more oxygen than a similarly-sized group of hardwood trees. Bamboo is a grass that does not require heavy chemical treatment to flourish, and unlike their hardwood counterparts, harvested bamboo fields maintain their root systems, which means less erosion and ultimately less chemical runoff.
Finally, bamboo products such as diapers, wipes, and packaging that will potentially end up in landfills, will biodegrade at much faster rates than will petroleum-based diapers, cotton wipes, and foam packaging. Throughout the life cycle, the advantages of using bamboo are clear. We, at Green Innovations Ltd, are pleased to offer to you the many benefits of being greener by living tree free with an expanding line of bamboo-based products
Accreditations

FDA - Food and Drug Administration
The FDA is an executive department agency of the United States Department of Health and Human Services. The FDA is established to be responsible for protecting and promoting public health through regulation of consumer products. The FDA is one of the most recognized certifications in the world.

EPA - Environmental Protection Agency
An agency of the United States federal government which has been established for the purpose of protecting human health and the environment. The EPA's purpose is to protect consumers from significant risk from human health and the environment on which they live learn and work.
 Nordic Environmental Label
Nordic Environmental Label
The Nordic Ecolabel is a comprehensive evaluation through the lifecycle of the product. This means that, in developing criteria, we look at the whole life cycle of the product and all its related environmental issues. Climate considerations are thus a key element of the assessment. Some criteria contain requirements linked directly to the climate, such as those concerning use of fossil fuels or energy consumption during the manufacturing process. The more important we judge the climate issue to be for a particular product group, the stricter and more extensive the requirements become in that area.
 BRC – British Retail Consortium
BRC – British Retail Consortium
The British Retail Consortium (BRC) is the lead trade association in the UK representing the whole range of retailers, from the large multiples and department stores through to independents, selling a wide selection of products through all levels of wholesale and retail. The BRC is a highly sought after certification in the UK as it is the authoritative voice of retail, recognized for its powerful campaigning and influence within government and as a provider of excellent retail information. The majority of UK, and many European and global retailers, and brand owners will only consider doing business with suppliers who have gained certification against the appropriate BRC Global Standard.
 ISO 9001:2000 – International Organization for Standardization
ISO 9001:2000 – International Organization for Standardization
ISO International Standards ensure that products and services are safe, reliable and of good quality, they are strategic tools that reduce costs by minimizing waste and errors and increasing productivity. They help companies to access new markets, level the playing field for developing countries and facilitate free and fair global trade.
 GMP – Good Manufacturing Practice
GMP – Good Manufacturing Practice
GMP is a production and testing practice that maintains and ensures quality control of a manufactured goods. Many countries have created uniform GMP guidelines to correspond with their own legislation. These GMP guidelines safeguard the consumer and ensuring the goods produced are of the highest quality.
GMP is enforced in the United States by the Food and Drug Administration (FDA).
The World Health Organization (WHO) version of GMP is used by pharmaceutical regulators and the pharmaceutical industry in over one hundred countries worldwide, primarily in the developing world. The European Union's GMP (EU-GMP) enforces similar requirements to WHO GMP, as does the FDA 's version in the US. Similar GMPs are used in other countries, with Canada, Japan, Singapore, Philippines and others having highly developed/sophisticated GMP requirements.
Featured Products
Toilet Rolls of 100% bamboo pulp
-
Cosmetic Wipes and Pads of 100% bamboo pulp
-
Facial Tissue of 100% bamboo pulp
-
Copy paper, notebooks and office papers of 100% bamboo pulp
-
Meat platters (bamboo-styrofoam) for ground beef, steaks, etc.
-
Cosmetic Wipes and Pads of 100% bamboo pulp
-
Facial Tissue of 100% bamboo pulp
-
Female Sanitary Pads
-
Panty Liners
Source: Green Innovations, OxBridge Research, Daily Stock Deals
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.




 OTCKing
OTCKing Feed Entries
Feed Entries
